
When it comes to infection prevention, most surgery center leaders focus on the use of sterile instruments, rigorous hand hygiene, and thorough environmental cleaning. Yet one overlooked risk remains right on the prep table: the antiseptic solutions your staff rely on every day.
Contrary to popular belief, antiseptics are not inherently sterile. The FDA and CDC have both reported contamination events associated with widely used antiseptics, including alcohol, chlorhexidine, benzalkonium chloride, and povidone-iodine.These outbreaks have led to product recalls, patient harm, and in some cases, systemic infections.
For surgery center administrators and executive leaders, this isn’t just a clinical issue—it’s a matter of operational safety, financial liability, and patient trust.
Why Non-Sterile Antiseptics Put Surgery Centers at Risk
Intrinsic Contamination
According to a recent BD whitepaper, some antiseptic solutions are already contaminated before they’re even opened. This can happen during manufacturing, particularly with non-sterile products that permit a small number of microbes to remain in the solution.
Extrinsic Contamination
Even if the antiseptic starts clean, improper handling after opening can introduce pathogens. Common risks include:
Sign up to get the latest industry news and offers right in your inbox
- Mixing on the back table
- Dilution of solutions
- Refilling bottles after initial use

The Consequences of Contamination
The microbes implicated in these contamination events aren’t harmless. Outbreaks have involved Bacillus cereus, Pseudomonas aeruginosa, Serratia marcescens, and Mycobacterium abscessus, among others. Infections linked to non-sterile antiseptics include:
- Wound infections
- Bacteremia
- Peritonitis
- Septic arthritis
- Systemic infections
For a surgery center, even a single contamination-related event can lead to extended stays, malpractice claims, reputational damage, and financial fallout.
What the FDA and CDC Say About Sterile Solutions

To protect patients, the FDA requires implantable and injectable products to meet a Sterility Assurance Level (SAL) of 10^-6, meaning only one viable microorganism is expected in one million sterilized units. While topical antiseptics may not always fall under the same requirement, the FDA and CDC recommend choosing sterile-labeled solutions whenever possible.
The biggest myth to break is that “antiseptics are self-sterilizing.” They are not. Without a validated sterilization process, antiseptics can harbor viable microbes and pose a real risk.
For leaders, the takeaway is clear: not all antiseptics are created equal, and making purchasing decisions based only on cost or availability can expose your center to avoidable risks.
Leadership Responsibility: Why This Matters for Surgery Center Administrators
As a surgery center leader, your role goes beyond managing staff and schedules. You’re responsible for ensuring a culture of safety that protects both patients and the bottom line. Here’s why antiseptic choice should be on your radar:
- Patient safety: Choosing sterile antiseptics reduces the risk of infection and safeguards surgical outcomes.
- Regulatory alignment: Staying in step with FDA and CDC guidance helps prevent compliance gaps.
- Financial stewardship: Avoids the high costs of infection-related readmissions, legal action, and product recalls.
- Operational excellence: Reinforces an infection prevention culture that builds staff confidence and patient trust.
In short, antiseptic sterility is not just a clinical detail—it’s a strategic leadership decision.
Infection Prevention Beyond Antiseptics
Of course, antiseptic choice is just one layer of protection. Surgery centers also need to evaluate how patients move through their facilities and how equipment contributes to or reduces contamination risks.

Every patient transfer introduces another opportunity for microbes to spread—from staff clothing, from equipment surfaces, or from cross-contact in shared spaces. By reducing the number of transfers and surfaces each patient comes into contact with, administrators can further strengthen infection prevention efforts.
Champion’s T5 Procedure Chair: Supporting Safer, Smarter Workflows
At Champion Healthcare Solutions, we believe equipment should work as hard as your staff to protect patients and streamline care. The T5 procedure chair was designed with that philosophy in mind.
Sterile antiseptics are one critical step toward stronger infection prevention. But minimizing cross-contamination risk also requires reducing unnecessary patient transfers and touchpoints—areas where the T5 delivers measurable impact.
One Patient, One Surface®
The T5 embodies a “one patient, one surface” approach: patients remain on the same surface from admission through procedure, imaging, transport, and discharge. This reduces transfers, minimizes touchpoints, and lowers the chance of contamination.
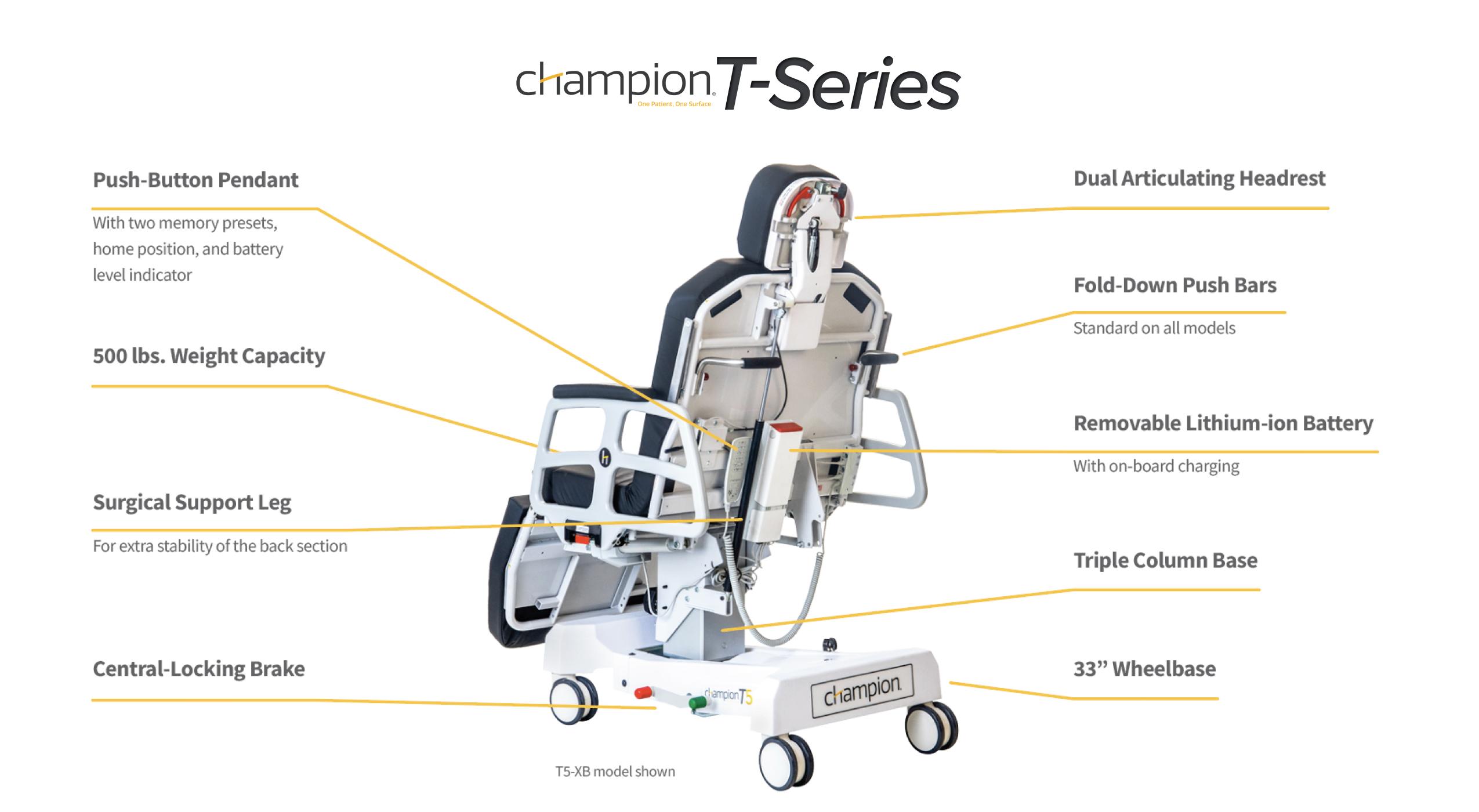
Clinical and Economic Advantages of the T5
- Powered positioning with memory presets eliminates repeated manual adjustments, improving throughput and reducing staff strain.
- Compact footprint requires half the space of a traditional stretcher, making it easier to navigate hallways, elevators, and surgical suites while freeing up storage.
- Low-profile, dual-articulating headpiece allows precise positioning—ideal for ophthalmology and plastic surgery applications.
- Advanced tilt capability (up to 20° Trendelenburg and 5° Reverse) supports surgical precision and patient safety.
- Redesigned lithium-ion battery system ensures consistent performance and easy on-board charging.
By pairing sterile antiseptics with workflow-driven equipment, such as the T5, surgery centers can enhance both infection prevention and operational efficiency—two of the most critical priorities for any administrator.
Discover the power of the T5 and check out our full T-Series lineup today. Then, connect with a Champion team member to see how our procedure chairs can enhance efficiency and safety in your surgery center.
